March 3 at 10:20 a.m.
Filed under:
Pharmaceuticals
By Associated Press
BioSante Pharmaceuticals Inc. said Thursday its potential pancreatic cancer treatment met key goals in a midstage study.
The company’s stock surged 32 cents, or 16 percent, to $2.35 in premarket trading.
The company said a newly published study showed the vaccine increased the median survival rate of patients by more than 25 percent to 24.8 months. The results were published in the February issue of the Annals of Surgery and involved 60 patients undergoing treatment at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center in Baltimore. Get the full story »
Nov. 15, 2010 at 9:25 a.m.
Filed under:
Pharmaceuticals,
Stock activity
By Reuters
A once-daily pill being developed by Bayer AG and Johnson & Johnson was better at preventing stroke than standard treatment, with less risk of the most worrisome types of bleeding, researchers said on Monday. Get the full story »
Oct. 8, 2010 at 3:20 p.m.
Filed under:
Earnings,
Pharmaceuticals,
Updated
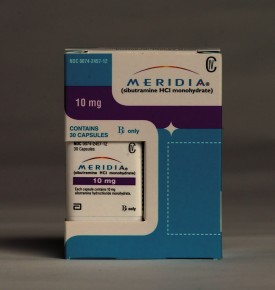 By Bruce Japsen and Andrew Zajac | Abbott Laboratories said Friday that it would withdraw the diet drug Meridia at the request of the U.S. Food and Drug Administration less than a month after it failed to win over one of the federal agency’s safety advisory panels.
By Bruce Japsen and Andrew Zajac | Abbott Laboratories said Friday that it would withdraw the diet drug Meridia at the request of the U.S. Food and Drug Administration less than a month after it failed to win over one of the federal agency’s safety advisory panels.
The FDA confirmed the North Chicago-based drug giant’s decision, saying Abbott withdrew the drug because of “clinical trial data indicating an increased risk of heart attack and stroke.” Get the full story »
July 26, 2010 at 3:22 p.m.
Filed under:
Government,
Pharmaceuticals
By Reuters
The Food and Drug Administration should better explain its reasons whenever it requires additional safeguards for risky drugs, a pharmaceutical industry group said Monday.
That recommendation is one of dozens expected this week at a public meeting on the FDA’s risk evaluation and mitigation strategies, or REMS, a set of tools to protect consumers from drugs with potentially serious side effects. Get the full story »





