The U.S. health regulator ordered painkiller makers to provide educational materials to help train physicians about the correct use of the drugs, as part of the Obama administration’s plan to tackle prescription drug abuse. Get the full story »
Johnson & Johnson
Visit our Filed page for categories. To browse by specific topic, see our Inside page. For a list of companies covered on this site, visit our Companies page.
J&J recalls more Tylenol bottles due to musty odor
Health care products maker Johnson & Johnson is recalling another lot of Tylenol products due to a musty odor which has already triggered five other recalls of the company’s over-the-counter medicines.
The latest recall involves more than 34,000 bottles of Tylenol 8 Hour Extended Release, which were distributed throughout the U.S. All of the bottles are from a single lot. Get the full story »
CEO of beleaguered J&J still got $29M in 2010
After a year in which Johnson & Johnson’s product quality control was deemed such a shambles that the U.S. government will oversee some plants, the board had praise for Chief Executive William Weldon and awarded him almost $29 million in overall compensation.
The once golden reputation of the diversified health care giant was tarnished by seemingly endless recalls of widely used consumer products as well as recalls of medical devices and products from other units in 2010. Get the full story »
FDA to oversee 3 troubled McNeil plants
Johnson & Johnson’s McNeil division, beset by an unstemmed tide of consumer product recalls, said Thursday that it had finalized a consent decree that would put three of its manufacturing facilities under supervision of U.S. health authorities.
McNeil said in a statement it had finalized the terms of a consent decree with the Food and Drug Administration for its facilities in Las Piedras, Puerto Rico, and Fort Washington and Lancaster, Pa. Get the full story »
Johnson & Johnson recalls insulin cartridges
Johnson & Johnson, which has been beset by a seemingly endless stream of product recalls, has recalled five lots of potentially leaky insulin pump cartridges that could lead to serious health problems and death.
It also has been warned by the Food and Drug Administration over manufacturing concerns for heart devices made at its Cordis unit’s San German, Puerto Rico facility. Get the full story »
FDA to toughen warnings on migraine drug
The U.S. Food and Drug Administration said it will strengthen warnings on the anti-migraine and anti-seizure treatment Topamax and its generic equivalents after new data suggested a higher risk for cleft palates in babies born to women taking the drug.
The move represents a setback for health-care products giant Johnson & Johnson, which owns Topamax maker Ortho-McNeil Pharmaceutical LLC. The subsidiary last May pleaded guilty to promoting the drug for off-label uses and had to pay an $81.5 million fine. Get the full story »
Beset by recalls, J&J cuts CEO’s bonus 45%
Johnson & Johnson cut the 2010 performance bonus of its chief executive, William Weldon, by 45 percent, from 2009 levels to $1.98 million, according to a regulatory filing.
The CEO’s diminished bonus comes after a year in which the company faced repeated recalls of consumer health products, medical devices and other products that hit its bottom line. Get the full story »
J&J recalls ‘pens’ that deliver Simponi doses
Johnson & Johnson has recalled at least 395 injection devices containing rheumatoid-arthritis drug Simponi in the U.S. and Germany, due to a potential defect that could result in an insufficient dose of the drug.
European health authorities warned Friday that the manufacturing snafu could cause a temporary shortage of the Simponi devices. As an alternative, patients are being advised to use prefilled syringes of Simponi. Get the full story »
Recalls squeeze Johnson & Johnson in 4Q
Johnson & Johnson’s revenue has slumped for a second straight year, prompting its chief executive to make an extraordinary pitch to soothe investors and defend the company’s handling of 17 costly recalls.
The health care giant, hammered by the weak global economy, growing pricing pressures and recalls that have kept many popular nonprescription medicines off store shelves, reported Tuesday a 12 percent profit decline and a 5.5 percent drop in sales for the fourth quarter. Get the full story »
J&J under fire for tampon supply problem
Johnson & Johnson, already under fire for a string of product recalls, has another public relations issue on its hands after its o.b. tampons temporarily disappeared from stores and little was said about what happened. Get the full story »
J&J recalls more Tylenol, other medications
Johnson & Johnson recalled nearly 47 million units of over-the-counter medicines Friday, the latest in a string of quality-related product recalls.
J&J said the latest recall resulted from a thorough examination of manufacturing records that the company had undertaken in the wake of earlier recalls. J&J plans to continue reviewing practices at additional plants and signaled further recalls could result. Get the full story »
FDA seeks to limit painkiller in Vicodin, others
U.S. health regulators are requesting a limit on the amount of acetaminophen in prescription pain medicines to curb the risk of liver damage.
The move announced on Thursday aims to limit combination drugs such as the opioids Percocet and Vicodin to 325 milligrams of acetaminophen per pill and calls for them to carry a “black box” warning about potential liver failure. Get the full story »
Cancer blood test coming to market
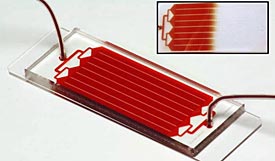
The HB-Chip can spot a single cancer cell lurking among a billion healthy ones . (AP Photo/PNAS Early Edition)
Includes updated information on the latest test format.
A blood test so sensitive that it can spot a single cancer cell lurking among a billion healthy ones is moving one step closer to being available at your doctor’s office.
Boston scientists who invented the test and health care giant Johnson & Johnson will announce Monday that they are joining forces to bring it to market. Four big cancer centers also will start studies using the experimental test this year. Get the full story »
Rep. Issa names source of tainted Rolaids
The incoming chairman of the U.S. congressional committee investigating Johnson & Johnson’s recalls of consumer products Friday identified the previously unnamed third-party manufacturer behind last week’s Rolaids recall.
Rep. Darrell Issa of California, in a letter to Food and Drug Administration Commissioner Margaret Hamburg, said investigations by his staff revealed North Carolina-based Best Sweet as the company contracted by J&J to produce widely used Rolaids antacid products. Get the full story »
Recall widens after metal, wood found in Rolaids
 Johnson & Johnson issued a recall of its Softchews Rolaids antacids Thursday after wood and metal bits were discovered in the tablets.
Johnson & Johnson issued a recall of its Softchews Rolaids antacids Thursday after wood and metal bits were discovered in the tablets.
J&J, which recalled some Rolaids products in November, said it was voluntarily recalling all lots of the Softchews products after potentially uncovering problems with a third-party manufacturer.
The recall is the latest in a string of pulled products for J&J’s consumers unit that has drawn attention from U.S. authorities and Congress, hurt sales for its consumer products parent company and tarnished J&J’s reputation. Get the full story »